Sticking electrons to a semiconductor with hydrazine creates an electrocatalyst
LOS ALAMOS, NM, June 13, 2016 – In the 2015 movie The Martian, stranded astronaut Matt Damon turns to the chemistry of rocket fuel, hydrazine and hydrogen, to create lifesaving water and nearly blows himself up. However, if you turn the process around and get the hydrazine to help, you create hydrogen from water by changing conductivity in a semiconductor, a transformation with wide potential applications in energy and electronics.
“We demonstrate in our study that a simple chemical treatment, in this case a drop of dilute hydrazine (N2H4) in water, can dope electrons directly to a semiconductor, creating one of the best hydrogen-evolution electrocatalysts,” said Gautam Gupta, project leader at Los Alamos National Laboratory in the Light to Energy team of the Lab’s Materials Synthesis and Integrated Devices group. The research was published in the journal Nature Communications.
Understanding how to use a simple, room-temperature treatment to drastically change the properties of materials could lead to a revolution in renewable fuel production and electronics. As part of the Los Alamos mission, the Laboratory conducts multidisciplinary research to strengthen the security of energy for the nation, work that includes exploring alternative energy sources.
In recent years, the materials science community has grown more interested in the electrical and catalytic properties of layered transition metal dichalcogenides (TMDs). TMDs are primarily metal sulfides and selenides (e.g., MoS2) with a layered structure, similar to graphite; this layered structure allows for unique opportunities, and challenges, in modifying electrical properties and functionality.
Gupta and Aditya Mohite, a physicist with a doctorate in electrical engineering, have been pioneering work at Los Alamos seeking to understand the electrical properties of TMDs and use that knowledge to optimize these semiconductors for renewable fuels production.
In this work, a MoS2 shell, MoOx core nanowires, as well as pure MoS2 particles and 2D sheets are tested for electrocatalysis of the hydrogen evolution reaction. The addition of dilute hydrazine to MoS2 significantly improves the electrocatalytic performance. Further characterization shows that the MoS2 changes from semiconducting behavior to having more metallic properties following the hydrazine exposure.
“The most interesting thing about this result is that it is different than conventional doping, where actual chemicals are added to a semiconductor to change its charge carrier concentration. In the case of hydrazine treatment, we are ‘doping’ electrons directly to the material, without modifying the original chemistry,” said Dustin Cummins, first author on this project, currently a postdoctoral researcher in the Laboratory’s Sigma Division working on the DOE/NNSA CONVERT Program, exploring fuel fabrication for next-generation reactors.
Cummins first found the hydrogen-production result working with Gupta at Los Alamos as a graduate student research affiliate from the University of Louisville (advisor: Dr. Mahendra Sunkara), and he continued to conduct experiments and refine discussion while working as a postdoc.
“Hydrazine acting as an electron dopant in inorganic semiconductors has been observed since the 1970s, but there is limited understanding of the process,” Cummins noted. “Our biggest hurdle was to prove to that hydrazine was actually changing the conductivity of the MoS2 system, and that is what results in increased catalytic activity, which was demonstrated on single-flake devices,” he said.
Multiple areas of Los Alamos staff expertise in layered semiconductors, chemistry, spectroscopy, electrical device fabrication and more all came together to provide some of the best understanding and mechanisms to date for hydrazine acting as an electron dopant.
This paper, “Efficient Hydrogen Evolution in Transition Metal Dichalcogenides via a Simple One-Step Hydrazine Reaction,” not only presents one of the best hydrogen water splitting electrocatalysts to date, but also “it opens up a whole new direction for research in electrochemistry and semiconductor device physics in general,” said Gupta.
Hydrogen production using zinc-doped carbon nitride catalyst irradiated with visible light
Recently, graphitic carbon nitride (g-C3N4) has been investigated as a photocatalyst for water splitting and organic dye degradation. In this study, we have developed a simple soft-chemical method of doping Zn into g-C3N4 to prepare a metal-containing carbon nitride. The doping was confirmed by x-ray photoelectron spectroscopy, and diffusion reflectance spectra revealed a significant red shift in the absorption edge of Zn/g-C3N4. This hybrid material shows high photocatalytic activity and good stability for hydrogen evolution from an aqueous methanol solution under visible light irradiation (420 nm). The hydrogen evolution rate was more than 10 times higher for a 10% Zn/g-C3N4 sample (59.5 mol) than for pure g-C3N4. The maximum quantum yield was 3.2% at 420 nm.
Hydrogen is an attractive energy source as a clean, cheap, renewable and a convenient alternative to fossil fuels. It is considered a feasible long-term solution for the global energy and environmental crises. Photocatalyst-assisted splitting of water is an ideal method of large-scale hydrogen production, as it can use solar energy sustainably and efficiently. To date, various semiconductors have been explored for water splitting; most of them are metal-based inorganic solids such as metal oxides, metal (oxy)sulfides and metal (oxy)nitrides. However, materials with appropriate bandgap and adequate stability for water splitting under visible light irradiation are still unavailable, and their development is considered a major challenge in photocatalysis research.
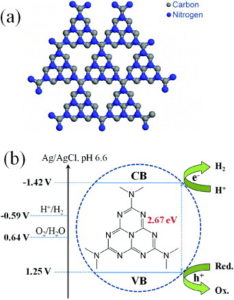
Recently, graphitic carbon nitride (g-C3N4) has showed promising performance as a metal-free polymeric photocatalyst for hydrogen production and organic dye degradation under visible light irradiation. This polymeric g-C3N4 material contains graphitic stacking of C3N4 layers, which are constructed from tri-s-triazine units connected by planar amino groups (scheme 1(a)). Pure g-C3N4 has a bandgap of 2.7 eV (460 nm), which originates from the sp2 hybridization of carbon and nitrogen atoms forming conjugated graphitic planes (scheme 1(b)). Because of the appropriate electronic band structure, nonmetallic g-C3N4 achieves the same function as conventional metal-based photocatalysts for H2 evolution from water, g-C3N4 exhibits high stability during photocatalysis in aqueous solutions, which might originate from the strong covalent bonds between carbon and nitrogen atoms.
Although g-C3N4 has shown great potential as a photocatalyst for water splitting, the estimated quantum efficiency remains low and needs to be improved. g-C3N4 structure contains nitrogen triangles having six lone-pair electrons, which are available for metal inclusion. Consequently, doping with metals should be a feasible method of designing new organic-metal hybrid materials based on the special structure of g-C3N4. Metal doping should alter the photochemical properties of g-C3N4, in particular, reduce the bandgap energy and expand light absorption into the visible range. Moreover, metallic species incorporated into the g-C3N4 framework can dope electrons to the g-C3N4 semiconductor.
Scheme 1 Schematic diagram of perfect g-C3N4 lattice constructed of graphitic stacking of C3N4 sheets made of tri-s-triazine units (a). Energy diagram of metal-free g-C3N4 photocatalyst for water splitting (b). The circled unit is a preferred site for metal inclusion.
Metal-containing carbon nitride compounds have been reported, and most of them showed good photocatalytic activities for the degradation of various organic dyes. However, there are only a few reports on photocatalytic water splitting using g-C3N4. In this study, we developed a simple soft-chemical method of doping g-C3N4 with zinc. Compared with other metals, Zn is easier to incorporate into g-C3N4 without destroying its graphite-like structure. Zinc in g-C3N4 captures photogenerated electrons from the conduction band and creates active sites for H2 evolution
Simple solution makes hydrogen production through solar water splitting more efficient and cheaper
Researchers from Delft University of Technology (TU Delft, The Netherlands), in collaboration with colleagues from the École polytechnique fédérale de Lausanne (EPFL, Switzerland), have found a simple yet effective solution to greatly increase the efficiency and stability of hydrogen production through solar-driven water splitting. By separating the positive and the negative electrodes using a bipolar membrane, they were able to create local optimal conditions for electrolysis. Furthermore, they achieved this using only Earth-abundant catalysts and solar cells, opening the way for more efficient and stable water-splitting systems at lower cost. They have published their findings in the journal Advanced Energy Materials.
Associate Professor Dr. Wilson Smith is driven in his research at TU Delft by the thought that one hours’ worth of sunlight reaching the Earth contains enough energy for one years’ worth of current energy demand worldwide. One of his challenges is being able to store and transport part of that energy for later use. So-called solar fuels could offer a solution, for example by harvesting solar energy and converting it into hydrogen by means of water splitting.
Electrolysis
Electrolysis is the process involved in this method. In order to realize efficient and long term water splitting, having a strong acid around the negative cathode and a strong base around the positive anode would be best. Up until now, most of the commercially available electrolysers run in either a strong acid or a strong base electrolyte. In these systems, the highly corrosive environments limit the choice of catalysts, and they suffer from the constraint of finding a suitable pair of electrodes for the one electrolyte. So far only precious, and therefore expensive, metal catalysts can do that job.
Simple solution
The seemingly simple solution of separating the two electrodes by a specialized membrane allows for optimization of the process by offering the electrodes their respective best environments. It also means that Earth-abundant catalysts can be used in the process, making it cheaper, more efficient, and more stable.
Efficient process
The international research team, including Dr. David Vermaas from TU Delft, has shown that using a bipolar membrane in this manner can lead to a water splitting system efficiency of 12.7%. Natural photosynthetic processes in plants run at about 1% efficiency, while an efficiency of 10% is considered to be the starting point for potential commercial viability, according to various techno economic analyses. An efficiency of 18% has been achieved for these types of processes, but only using precious metals and other very expensive (and unstable) materials. To be able to achieve this high efficiency while also using all-Earth-abundant components in the solar cell and catalysts, makes the achievement an excellent demonstration for this technology. Dr. Smith says, ‘This is a strong scientific step that can help the transition from lab scale systems into practical devices.’
Potential for other applications
The principle of separating the poles in these types of cells also looks very promising for other applications, according to Dr. Smith. ‘Using this bipolar membrane for electrochemical systems, we are now able in theory to click together the optimal half reaction components for processes like pieces of Lego. This has a huge potential for other electrochemical reactions, such as the production of ammonia and hydrocarbons, while separating the oxidation half reaction completely. In this next step, we can finally replicate nature and make a truly artificial photosynthetic system that goes well beyond the efficiencies in nature.’